Strong metal–support interactions (SMSI) describes the phenomenon that platinum group metals (PGMs) supported on reducible oxides lose their capability to adsorb small molecule after high-temperature reduction. SMSI has significant influence on the activity, selectivity and stability of catalysts.
Scientists discovered a few novel SMSI effects of gold and PGMs nanocatalysts. However, whether SMSI can form on single-atom catalysts (SACs) or not remains unclear.
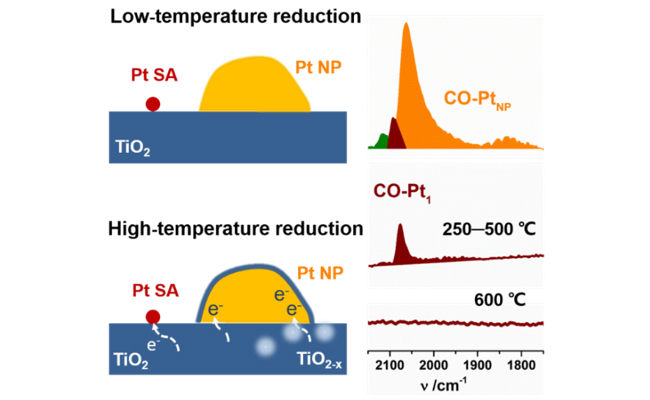
Recently, Prof. QIAO Botao and Prof. ZHANG Tao's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences discovered that SMSI could occur on TiO2-supported Pt single atoms but at a much higher reduction temperature than that for Pt nanoparticles (NPs).
Furthermore, the suppression of CO adsorption on Pt single atoms, different from that of Pt NPs, originated from coordination saturation rather than the physical coverage.
Pt single atoms vs NPs: difference in strong-metal support interactions. (Image by HAN Bing and GUO Yalin)
The researchers prepared Pt/TiO2 catalyst by a modified photochemical method, consisting of both Pt single atoms and NPs. They found that Pt NPs lost adsorption ability at 250 °C reduction. In contrast, CO adsorption on Pt single atoms only disappeared completely at reduction at as high as 600 °C. The followed 300 °C re-oxidation could restore CO adsorption on both NPs and single atoms.
They performed low-energy ion scattering (LEIS) to detect the change of surface Pt atoms and demonstrated that Pt single atoms neither were encapsulated nor sank into the support after high temperature reduction.
Theoretical calculations showed that the reason for Pt single atoms losing their adsorption capacity was coordination saturation (18-electron rule) rather than the physical coverage of Pt atoms by the support. According to this finding they demonstrated that in hydrogenation of 3-nitrostyrene, the single-atom sites played a major role while the contribution of NP sites was negligible by selectively encapsulating the NPs sites.
The study not only demostrates for the first time that the SMSI between Pt single atoms and TiO2 support can occur, but also reveals the difference of SMSI in single atoms and NPs.
These findings help us deeply understand SMSI effect, and provide an effective approach to study catalytic active centers and tuning catalytic performance.
The study was published as a hot paper in Angewandte Chemie International Edition. It was supported by the National Natural Science Foundation of China, National Key Projects for Fundamental Research and Development of China, Strategic Priority Research Program of the Chinese Academy of Sciences, LiaoNing Revitalization Talents Program and DNL Cooperation Fund, CAS.
Moreover, this paper was dedicated to the 70th anniversary of Dalian Institute of Chemical Physics, Chinese Academy of Sciences. (Text by HAN Bing and GUO Yalin)